KEY POINTS
- Iron is an essential micronutrient in the diet, but commonly, athletes often present as iron deficient.
- Several mechanisms are associated with iron deficiency in athletes, including gastrointestinal bleeding, sweating, hemoglobinuria, red blood cell hemolysis, low energy intake, diet restriction, and for menstruating female athletes – menstrual blood loss.
- There are three stages of iron deficiency according to severity of condition. Standardization of blood collection is required to ensure determination of iron stores is accurate.
- There are three key approaches to treating an iron deficiency: (i) increasing dietary iron intake; (ii) oral iron supplementation; or (iii) intravenous iron administration.
- The approach taken to treat iron deficiency should consider the severity of the iron deficit from biochemical and dietary assessments.
INTRODUCTION
Iron is an essential micronutrient in our diet that contributes to several central roles of relevance to exercise and athletic performance. For instance, iron is fundamental to the healthy production of red blood cells, which are essential for oxygen transport, and therefore, the aerobic capacity of an individual. Further, iron plays a critical role in the mitochondria via the electron transport chain, meaning there is a link between this micronutrient and energy (adenosine triphosphate) production. Collectively, oxygen supply and mitochondrial energy production are the cornerstones of aerobic activity, and therefore, it makes sense that healthy iron stores are essential to optimal athletic pursuit. However, iron deficiency is the most common nutrient disorder reported globally, and in various athlete cohorts, is suggested to impact ~15-35% of females and ~5-11% of males (Sim et al., 2019).
The high incidence of iron deficiency in athlete populations is likely a result of a complex combination of mechanisms that co-exist to create both acute situations of iron loss, in addition to difficulties with consuming/absorbing sufficient amounts of iron from the diet to support increased demands (i.e., for replacement and adaptation). Such acute mechanisms of iron loss may include gastrointestinal bleeding, sweating, hemoglobinuria, and red blood cell hemolysis, which can occur from a combination of fluid loss, dehydration, and the mechanical stress of exercise itself (for review see Peeling et al., 2008). Furthermore, difficulties in absorbing iron may result from exercise-induced alterations in hormones that regulate iron uptake in the gut (Peeling et al., 2009), whereas difficulties in consuming sufficient amounts of iron can be related to low energy availability (LEA). Here, insufficient caloric intake occurs relative to the athlete’s energy requirements, which associates with a reduction in the amount of iron consumed in the diet (Petkus et al., 2017). Finally, in female athletes, the hormone profile of a natural menstrual cycle may have some impact on the regulation of iron absorption (Kim et al., 1993; Laine et al., 2016), while blood loss from menses also plays a significant role in iron loss. These latter two mechanisms likely explain the higher prevalence of iron deficiency in female athletes compared to males (Coad & Conlon, 2011). Collectively, it is this myriad of factors that, in combination, appear to have a negative impact on an athlete’s iron stores over time.
The purpose of this Sports Science Exchange (SSE) article is to initially explore the impact of exercise on iron regulation and absorption, before establishing the current approaches to defining an iron deficiency in athlete populations. Subsequently, the various treatment options available to improve the iron status of an athlete are discussed, providing recommendations that align with the severity of the issue. Ultimately, the hope is to provide both athletes and practitioners with a useful guide to help navigate the complex issue of iron deficiency in sport.
EXERCISE AND THE REGULATION OF IRON ABSORPTION
Iron absorption from the gut is regulated by the hormone hepcidin, which undergoes homeostatic regulation according to the iron needs of the body (Nemeth et al., 2004). Hepcidin interacts with the body’s iron export cells, Ferroportin, causing a degradation of the channel, which inhibits the ability to transfer iron across the gut and into circulation (Nemeth et al., 2004). When the iron needs of the body are low (i.e., high iron stores), hepcidin levels are elevated to prevent excessive iron entry; conversely, when the iron needs of the body are high (i.e., low iron stores), hepcidin levels are suppressed to encourage intestinal iron uptake (Nemeth et al., 2004). Despite this seemingly well-regulated process, there are additional factors at play that can also influence circulating hepcidin levels, such as inflammatory cytokines (namely interleukin-6; IL-6) (Kemna et al., 2005) and hypoxic-driven pathways (Hintze & McClung, 2011). Interestingly, increases in IL-6 have been shown to directly increase the circulating levels of hepcidin. This was initially shown in rodents injected with lipopolysaccharide (LPS; to stimulate inflammation), where a significant elevation in IL-6 was followed by a significant rise in hepcidin levels 3 h later; a response that was not replicated in IL-6 knockout rodents receiving the identical LPS injection (Kemna et al., 2005).
Such findings are relevant to exercise settings, since it is well established that an exercise stimulus results in a transient increase to IL-6 levels in the immediate post-exercise period (Pedersen, 2000). This knowledge led to early work from our research team, to determine if the exercise-driven increases in IL-6 were associated with an increase in hepcidin levels, similar to that seen in the rodent models. In our repeated measures, crossover study design we had male and female athletes either running on a treadmill for 60 min or sitting in the lab for the same time period at rest (Peeling et al., 2009). Immediately prior to this exercise (or rest) stimulus, and then immediately post, and at the 3, 6, and 24 h post-exercise time points, we measured circulating levels of IL-6 and hepcidin to see the impact of exercise on acute iron regulation. Our data showed that, compared to both the resting condition and baseline (pre-exercise), IL-6 was significantly increased immediately after exercise, whereas hepcidin levels were significantly elevated at both 3 and 6 h post-exercise. Since this work back in 2009, a plethora of studies have replicated these findings, and it is now well established that significant transient increases in hepcidin levels occur 3 h after the completion of an exercise bout (Dahlquist et al., 2017; McCormick et al., 2019; Newlin et al., 2012). These findings raise interesting questions relevant to the timing of food intake around exercise since we know that the role of hepcidin is to decrease the absorption of iron from the gut; accordingly, more recent research has sought to explore the impact of hepcidin elevations on iron absorption using isotopic tracers to follow iron metabolism in the post-exercise period (Barney et al., 2022; McCormick et al., 2019). This research is explored in more detail in the sections below.
Interestingly, our work has also shown that the transient increase in hepcidin levels at 3 h post-exercise is not evident in athletes considered to be iron deficient (serum ferritin <30 μg/L), despite incurring an increase in post-exercise IL-6 (Peeling et al., 2014). This outcome shows a competing mechanism to the IL-6 story, such that, the body’s ability to sense its iron needs can overcome the impact of an elevated inflammatory response to encourage iron uptake at the level of the gut. This is a positive body response for those already suffering from iron deficiency, however, the outcome is not the same for those considered to have ‘borderline’ iron stores. In this group, the data showed that hepcidin elevations occur in individuals with serum ferritin in the range of 30-50 μg/L (Peeling et al., 2014), which is considered ‘suboptimal’ iron stores in athletes who are challenging the body to adapt to a training stimulus. Furthermore, when an iron-deficient athlete is trying to replete iron stores through oral iron supplementation, it is common that their iron stores only improve to a level that brings them back into the ‘suboptimal’ range. Accordingly, athletes presenting with iron stores in the ‘sub-optimal’ range are potentially in a perpetual cycle of falling in and out of iron-deficient states, which can begin to impact training consistency and performance over time. This isn’t to say that the issue cannot be addressed through supplementation; however, it does highlight that the treatment of iron deficiency can be slow and requires routine monitoring to assess the efficacy of treatment approach.
Given these issues, there is a warranted focus on the testing of athletes’ iron status and the approaches to iron intake and supplementation for optimal efficacy. Accordingly, the remainder of this SSE article will focus on the contemporary and practical approaches to iron status assessment and repletion of iron stores in deficient athletes.
TESTING IRON LEVELS AND THRESHOLDS OF INTERPRETATION
In 2019, our research team developed an athlete-specific iron screening framework (Sim et al., 2019) to assist coaches, athletes, and clinicians by highlighting: (i) specific factors that increase an athlete’s risk for iron deficiency; (ii) cut points for hematological parameters; and (iii) strategies to improve the accuracy of iron deficiency assessments. This information is summarized in Figure 1 and collectively provides guidelines around how often an athlete’s iron levels should be assessed.
Currently, there is debate about which hematological variables should be used to assess an athlete's iron status, with several established and emerging biomarkers available for consideration (see Clenin et al., 2015). However, when considering athletic populations, we propose that the minimum routine clinical assessment for iron deficiency should include analysis of serum ferritin (sFer), hemoglobin concentration (Hb), and transferrin saturation (TSAT) (Peeling et al., 2007; Sim et al., 2019). These iron indices provide an indication of current iron stores (sFer), red blood cell levels (Hb), and transport (TSAT), which are critical in the determination of functional iron status. Utilizing these biomarkers, three stages of iron deficiency have been proposed in athletic populations (Peeling et al., 2007) (Figure 1).
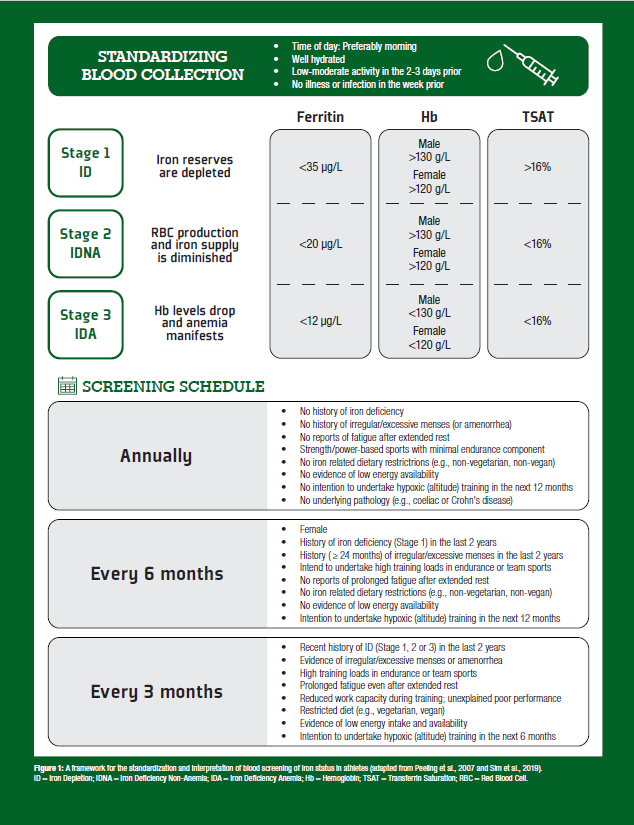
Stage 1 (iron depletion; ID) is characterized by depleted sFer with no noticeable change to Hb or TSAT. Left untreated, depleted iron stores can progress towards further compromise of the iron stores, and a reduction in the iron bound to transferrin (stage 2: iron deficiency non-anemia; IDNA), although Hb at this stage remains sufficient. Progression of the condition from this point results in compromised red blood cell production, resulting in impaired Hb levels, indicating that Stage 3 iron deficient anemia (IDA) has been reached. Current literature suggests that work capacity and athlete performance are primarily affected only when an athlete reaches stage 3 (IDA), when oxygen transport is substantially diminished (Myhre et al., 2015); whereas the impact of ID and IDNA on performance remains equivocal (Burden et al., 2015; Rubeor et al., 2018). However, the negative effects of IDNA (stage 2) may be associated with impaired function of oxidative enzymes, respiratory proteins, immune function, and perceptions of fatigue, which can collectively impair performance (Burden et al., 2015), especially in high-performance athletes where optimizing the factors that impact adaptation are critical. Accordingly, early identification through the proposed screening protocol is essential, as early correction of iron depletion is likely to prevent the issue from further progressing into stages 2 and 3. Of note, supplementing iron to non-deficient populations does not improve performance (see Sim et al., 2019, for review), and in fact, having too much iron in the system can be toxic (Papanikolaou & Pantopoulos, 2005), which makes the concept of ‘more is better’ irrelevant to iron supplementation.
Of importance, blood collection standardization is imperative to improve the accuracy of assessing an athlete's iron status. Further, training load in the days prior to blood assessment should be controlled and considered. Specifically, any training with the potential to induce higher levels of muscle damage/inflammation (e.g., eccentric exercise) should be avoided where possible. This is especially relevant for sFer, which is known to be elevated as part of the acute phase response, especially after intense training, or when an athlete has had an illness (Fallon et al., 2001). Importantly, changes in plasma volume can impact Hb, and if not considered, may present as pseudo-anemia (or sports anemia), which can provide a distorted picture of an athlete’s current iron status. Ideally, blood collection should occur in the morning, after a day of rest before training resumes, with the athlete in a healthy and hydrated state after an overnight fast (Sim et al., 2019).
TREATMENT OPTIONS FOR IRON SUPPLEMENTATION
When an athlete has been identified with compromised iron stores, the practitioner (i.e., dietician or sports physician) must then work with them to decide on the best approach to help fix the problem. Typically, there are three approaches to treating an iron deficiency. These include (i) an increase in dietary iron intake (also known as a ‘food first approach’); (ii) oral iron supplementation; or (iii) intravenous iron infusions. Generally, the approach taken will depend on the severity of the iron deficit, and the decision made should consider the outcomes of the blood screening results according to the framework outlined above. In cases of ID, an initial approach of increasing dietary iron intake might be considered, especially if any associated nutritional assessment shows an overall LEA or nutrient deficit in the diet. However, if an increase in dietary iron intake is not possible through food alone, the addition of a dietary iron supplement might be the next step considered in the decision-making process. Of note, both an increase in dietary iron intake and an oral iron supplement in combination might be used in cases of stage 2 IDNA. Finally, in persistent and unresponsive cases, where stage 3 IDA has been reached, the use of an iron infusion might be considered; however, this parenteral approach should only be undertaken in consultation with a trained sports physician.
Increasing Dietary Iron Intake
As a first-line approach to improving iron stores, practitioners working with iron-compromised athletes should consider a dietary assessment to explore the athlete’s overall energy intake, their consumption of key micronutrients, the combinations of foods they eat, and importantly, the timing of their nutrient consumption. In combination, these factors are important to know in the context of iron regulation, with dietary analysis potentially useful for highlighting areas of concern relevant to iron intake and absorption. For instance, LEA is common in athlete populations with high training volumes and/or in weight-sensitive sports where physique is associated with success, whereby energy intake is not sufficient to meet energy demands (Wasserfurth et al., 2020). In such settings, a concurrent reduction in the intake of micronutrients (including iron) is common, and therefore, an assessment of overall energy intake may reveal opportunities to increase iron through greater overall nutrient consumption. A dietary assessment may also highlight the sources of iron consumed within an athlete’s diet, which may indicate the amounts of heme (animal tissue) and non-heme (plant source) iron present. Interestingly, heme iron shows much greater efficiency of absorption from the diet as compared to non-heme sources (i.e., 5-35% of heme iron in food is absorbed, as compared to only 2-20% of non-heme) (Beard & Tobin, 2000). Heme iron is typically found in animal tissue, whereas non-heme iron is in plant sources. Accordingly, it is the differences in absorption potential between heme and non-heme iron that likely explains the higher prevalence of iron deficiency in diet-restricted athletes (i.e., vegetarians) as compared to those eating a mixed diet of both animal and plant sources (Pawlak et al., 2018). In addition to iron source, a dietary assessment might also consider the combinations of food being consumed together. Of note, the are several inhibitors (i.e., calcium, polyphenols, tannins) and enhancers (i.e., Vitamin C) of iron absorption in the diet, which should be considered (i.e., avoided or exploited) when making decisions on what foods to consume when trying to maximize iron uptake from a given meal (Hurrell & Egli, 2010).
Finally, the timing of iron intake should also be explored as part of the dietary assessment, with a specific focus on the time of day that high iron-containing foods are consumed (since hepcidin concentrations show a diurnal increase over the day, Troutt et al., 2012), and the proximity of iron intake relative to exercise (Barney et al., 2022; McCormick et al., 2019). Such considerations may be important for optimizing iron uptake since work from our group has shown that fractional iron absorption (measured using stable iron isotopes) is increased in the morning as compared to the afternoon, and when consumed within 30 min of completing exercise (McCormick et al., 2019). Interestingly, both the post-exercise IL-6 (immediate) and hepcidin (3 h post) levels were elevated in our study. However, it appears that if consumed within a short window of time after exercise cessation (before the peak in hepcidin levels), athletes can optimize the uptake of iron from a single meal. In support of this concept, a 36% decrease in fractional iron absorption (also using stable iron isotopes) was seen when iron was consumed at 2 h post-exercise (as compared to a resting control trial), timed to coincide with the 3 h post-exercise peak in hepcidin concentrations (Barney et al., 2022). Accordingly, contemporary thinking on optimizing iron absorption from dietary intake would suggest we consume our richest iron-containing meals in the morning, and if exercise training occurs, within 30 min of completing the session.
Oral Iron Supplements
Oral iron supplements are the most common approach used to address an iron deficiency. However, as suggested in the name, this option should be considered as supplemental support to an increase in dietary iron intake, which has been considered in the context of a dietary analysis exploring the impact of iron source, consumption timing, and concurrent food intake on absorption. Oral iron supplements come in many forms, ranging in terms of dosage, formulation (pill vs. liquid; slow vs. quick release), and chemical state (ferrous or ferric iron form) (Santiago, 2012). However, ferrous sulphate tablets are the most common approach taken for the oral treatment of iron deficiency. Typically, oral iron treatment regimens consist of a daily iron supplement, which ranges in elemental iron content from 60-200 mg. Generally, the lower dose approach is used for individuals with low gastrointestinal (GI) tolerance to the supplement (Rimon et al., 2005), and the higher dosages used for severe deficiency (i.e., stage 3 IDA), or in short bursts for athletes with low iron stores looking to support an upcoming sojourn to altitude (Govus et al., 2015; Stellingwerff et al., 2019). Commonly, however, a daily dose of ~100 mg is consumed, usually in combination with an additional source of Vitamin C to help increase absorption. Such an approach is well established to increase sFer levels by 30-50% over a 6-8 week period (Dawson et al., 2006; McCormick et al., 2020).
Of consideration in approaches to restoring an individual’s iron status is the commonly incurred GI upset that has been attributed to the regular consumption of ferrous sulphate. Such negative side effects can reduce compliance and commitment to the supplement protocol, and in severe cases, can negatively affect an athlete’s ability to train effectively. Accordingly, athletes experiencing GI distress from oral iron supplements should consider: (a) reducing the iron content in the supplement (i.e., 60 mg) (Rimon et al., 2005); (b) consuming an alternative or new generation formulation (i.e., enteric-coated, maltodextrin combined, sucrosomial iron, etc.) (Cancelo-Hidalgo et al., 2013; Elli et al., 2018); or (c) reducing the frequency of intake (i.e., to alternate days) (McCormick et al., 2020; Stoffel et al., 2020). Recent work from our group (McCormick et al., 2020) has shown that consuming 105 mg elemental iron on alternate days was equally as effective in increasing sFer levels (~60% increase) as daily consumption, despite only 50% of the total iron content being consumed in the alternate condition (2,914 vs. 5,824 mg over 8 weeks). Accordingly, an alternate-day oral iron supplement protocol in athletes appears to be a viable, economical, and gut-friendly approach to increasing iron stores.
Intravenous Iron Infusions
Despite the established efficacy and consistent outcome of oral iron supplementation, it is clear that the process is relatively slow (i.e., 8+ weeks), and if sFer stores are extremely low, the impact of oral supplementation is relatively small (i.e., a 70% increase in an IDA athlete with sFer of 12 μg/L only increases levels to 20 μg/L, still rendering them in stage 2 IDNA – assuming Hb also improved with treatment). Accordingly, in severe and unresponsive to oral supplementation cases of IDA, consideration might be given to the use of intravenous (IV) iron, as a rapid and effective means of bypassing the gut and delivering iron directly to the circulation. Of note, a 300-500 mg dose of IV iron can increase sFer levels by 200-400% (Garvican et al., 2014), with peak ferritin concentrations occurring after 7–9 days and Hb increasing within 2–3 weeks (Baird-Gunning & Bromley, 2016). However, literature also suggests that the impact of IV iron treatment is only effective in increasing Hb in populations where anemia is present, with low to very low-quality of evidence to suggest any positive impact on IDNA (Miles et al., 2019). Therefore, IV approaches to iron treatment should be reserved for severe and persistent cases of IDA in athlete populations, with any decision to undertake this treatment method taken in conjunction with a trained sports physician, given the complexities of the World Anti-Doping Agency (WADA) rules that exist in relation to the various methods of delivery (for details, see Sports Integrity Australia, 2019).
PRACTICAL APPLICATIONS
- Routine testing of an athlete’s iron stores should occur according to the iron assessment framework set out in Figure 1.
- Dietary assessment of an athlete with iron deficiency should explore the overall energy intake, the diet’s composition of iron, the combinations of foods eaten with high iron-containing foods, and the timing of iron intake in relation to both time of day and proximity to exercise.
- If a dietary approach is deemed suitable, a food-first approach to increasing iron intake should include:
- Matching energy intake with energy demand.
- Ensuring a balance of heme and non-heme iron food sources exist in the diet and maximizing non-heme iron intake in non-meat eating populations.
- Maximizing the co-consumption of iron absorption enhancers (Vitamin C) and minimizing the co-consumption of iron absorption inhibitors (calcium, polyphenols, etc.) when iron-rich foods are consumed.
- Consuming iron-rich meals in the morning, and if training occurs, within 30 min of exercise cessation.
- If additional dietary iron is needed, oral iron supplements should be used to complement the treatment strategy. Considerations for oral iron supplements should include:
- Consuming one ferrous sulphate tablet daily, containing ~100 mg of elemental iron, for an 8-week period before reassessment of blood results.
- In cases where GI upset prevails, consider reducing the daily dose to 60 mg elemental iron, using an enteric-coated or slow-release formula, or reducing the overall dosage by only consuming the supplement every second day.
- In severe and persistent cases of IDA, discuss the option of IV iron treatment with your sports physician. However, be aware of the current WADA rules regarding administration of IV iron products.
SUMMARY
Despite iron being an essential micronutrient in the diet, athletes commonly present as iron deficient. A myriad of mechanisms are associated with iron deficiency in athletes, and although their individual impact on iron loss is likely small from any single exercise session, it is the interaction and accumulative effect over time that likely influences overall iron status. Understanding the mechanisms of iron loss in relation to exercise is important in the strategic approach to repleting and maintaining healthy iron stores since this knowledge can help us best determine how and when to consume our high iron-containing meals and/or iron supplements. A key to doing this effectively is to ensure athletes are working with trained sports dietitians and sports physicians as part of their nutritional assessment and treatment plans.
The views expressed are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc
REFERENCES
Baird-Gunning, J., and J. Bromley (2016). Correcting iron deficiency. Aust. Prescr. 39:193- 199.
Barney, D.E., J.R. Ippolito, C.E. Berryman, and S.R. Hennigar (2022). A prolonged bout of running increases hepcidin and decreases dietary iron absorption in trained female and male runners. J. Nutr. 152:2039-2047.
Beard, J., and B. Tobin (2000). Iron status and exercise. Am. J. Clin. Nutr. 72(2 Suppl):594S- 597S.
Burden, R.J., K. Morton, T. Richards, G.P. Whyte, and C.R. Pedlar (2015). Is iron treatment beneficial in, iron-deficient but non-anaemic (IDNA) endurance athletes? A systematic review and meta-analysis. Br. J. Sports Med. 49:1389-1397.
Cancelo-Hidalgo, M.J., C. Castelo-Branco, S. Palacios, J. Haya-Palazuelos, M. Ciria- Recasens, J. Manasanch, and L. Perez-Edo (2013). Tolerability of different oral iron supplements: a systematic review. Curr. Med. Res. Opin. 29:291-303.
Clenin, G., M. Cordes, A. Huber, Y.O. Schumacher, P. Noack, J. Scales, and S. Kriemler (2015). Iron deficiency in sports - definition, influence on performance and therapy. Swiss Med. Wkly. 145:w14196.
Coad, J., and C. Conlon (2011). Iron deficiency in women: assessment, causes and consequences. Curr. Opin. Clin. Nutr. Metab. Care 14:625-634.
Dahlquist, D.T., T. Stellingwerff, B.P. Dieter, D.C. McKenzie, and M.S. Koehle (2017). Effects of macro- and micronutrients on exercise-induced hepcidin response in highly trained endurance athletes. Appl. Physiol. Nutr. Metab. 42:1036-1043.
Dawson, B., C. Goodman, T. Blee, G. Claydon, P. Peeling, J. Beilby, and A. Prins (2006). Iron supplementation: oral tablets versus intramuscular injection. Int. J. Sport Nutr. Exerc. Metab. 16:180-186.
Elli, L., F. Ferretti, F. Branchi, C. Tomba, V. Lombardo, A. Scricciolo, L. Doneda, and L. Roncoroni (2018). Sucrosomial iron supplementation in anemic patients with celiac disease not tolerating oral ferrous sulfate: A prospective study. Nutrients 10:330.
Fallon, K.E., S.K. Fallon, and T. Boston (2001). The acute phase response and exercise: court and field sports. Br. J. Sports Med. 35:170-173.
Garvican, L.A., P.U. Saunders, T. Cardoso, I.C. Macdougall, L.M. Lobigs, R. Fazakerley, K.E. Fallon, B. Anderson, J.M. Anson, K.G. Thompson, and C.J. Gore (2014). Intravenous iron supplementation in distance runners with low or suboptimal ferritin. Med. Sci. Sports Exerc. 46:376-385.
Govus, A.D., L.A. Garvican-Lewis, C.R. Abbiss, P. Peeling, and C.J. Gore (2015). Pre-altitude serum ferritin levels and daily oral iron supplement dose mediate iron parameter and hemoglobin mass responses to altitude exposure. PLoS One 10:e0135120.
Hintze, K.J., and J.P. McClung (2011). Hepcidin: A critical regulator of iron metabolism during hypoxia. Adv. Hematol. 2011:510304.
Hurrell, R., and I. Egli (2010). Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 91:1461S-1467S.
Kemna, E., P. Pickkers, E. Nemeth, H. van der Hoeven, and D. Swinkels (2005). Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. Blood 106:1864-1866.
Kim, I., E.A. Yetley, and M.S. Calvo (1993). Variations in iron-status measures during the menstrual cycle. Am. J. Clin. Nutr. 58:705-709.
Laine, F., A. Angeli, M. Ropert, C. Jezequel, E. Bardou-Jacquet, Y. Deugnier, V. Gissot, K. Lacut, S. Sacher-Huvelin, A. Lavenu, B. Laviolle, and E. Comets (2016). Variations of hepcidin and iron-status parameters during the menstrual cycle in healthy women. Br. J. Haematol. 175:980-982.
McCormick, R., D. Moretti, A.K.A. McKay, C.M. Laarakkers, R. Vanswelm, D. Trinder, G.R. Cox, M.B. Zimmerman, M. Sim, C. Goodman, B. Dawson, and P. Peeling (2019). The impact of morning versus afternoon exercise on iron absorption in athletes. Med. Sci. Sports Exerc. 51:2147-2155.
McCormick, R., A. Dreyer, B. Dawson, M. Sim, L. Lester, C. Goodman, and P. Peeling (2020). The effectiveness of daily and alternate day oral iron supplementation in athletes with suboptimal iron status (Part 2). Int. J. Sport Nutr. Exerc. Metab. 30:191-196.
Miles, L.F., E. Litton, G. Imberger, and D. Story (2019). Intravenous iron therapy for non-anaemic, iron-deficient adults. Cochrane Database Syst. Rev. 12:CD013084.
Myhre, K.E., B.J. Webber, T.L. Cropper, J.N. Tchandja, D.M. Ahrendt, C.A. Dillon, R.W. Haas, S.L. Guy, M.T. Pawlak, and S.P. Federinko (2015). Prevalence and impact of anemia on basic trainees in the US air force. Sports Med. Open 2:23.
Nemeth, E., M.S. Tuttle, J. Powelson, M.B. Vaughn, A. Donovan, D.M. Ward, T. Ganz and J. Kaplan (2004). Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090-2093.
Newlin, M.K., S.Williams, T. McNamara, H. Tjalsma, D.W. Swinkels, and E.M. Haymes (2012). The effects of acute exercise bouts on hepcidin in women. Int. J. Sport Nutr. Exerc. Metab. 22:79-88.
Papanikolaou, G., and K. Pantopoulos (2005). Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 202:199-211.
Pawlak, R., J. Berger, and I. Hines (2018). Iron status of vegetarian adults: A review of literature. Am. J. Lifestyle Med. 12:486-498.
Pedersen, B.K. (2000). Special feature for the Olympics: effects of exercise on the immune system: exercise and cytokines. Immunol. Cell Biol. 78:532-535.
Peeling, P., T. Blee, C. Goodman, B. Dawson, G. Claydon, J. Beilby, and A. Prins (2007). Effect of iron injections on aerobic-exercise performance of iron-depleted female athletes. Int. J. Sport Nutr. Exerc. Metab. 17:221-231.
Peeling, P., B. Dawson, C. Goodman, G. Landers, and D. Trinder (2008). Athletic induced iron deficiency: new insights into the role of inflammation, cytokines and hormones. Eur. J. Appl. Physiol. 103:381-391.
Peeling, P., B. Dawson, C. Goodman, G. Landers, E.T. Wiegerinck, D.W. Swinkels, and D. Trinder (2009). Effects of exercise on hepcidin response and iron metabolism during recovery. Int. J. Sport Nutr. Exerc. Metab. 19:583-597.
Peeling, P., M. Sim, C.E. Badenhorst, B. Dawson, A.D. Govus, C.R. Abbiss, D.W. Swinkels, and D. Trinder (2014). Iron status and the acute post-exercise hepcidin response in athletes. PLoS One 9:e93002.
Petkus, D.L., L.E. Murray-Kolb, and M.J. De Souza (2017). The unexplored crossroads of the female athlete triad and iron deficiency: A narrative review. Sports Med. 47:1721-1737.
Rimon, E., N. Kagansky, M. Kagansky, L. Mechnick, T. Mashiah, M. Namir, and S. Levy (2005). Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am. J. Med. 118:1142-1147.
Rubeor, A., C. Goojha, J. Manning, and J. White (2018). Does iron supplementation improve performance in iron-deficient nonanemic athletes? Sports Health 10:400-405.
Santiago, P. (2012). Ferrous versus ferric oral iron formulations for the treatment of iron deficiency: a clinical overview. Scientific World J. 2012:846824.
Sim, M., L.A. Garvican-Lewis, G.R. Cox, A. Govus, A.K. McKay, T. Stellingwerff, and P. Peeling (2019). Iron considerations for the athlete: a narrative review. Eur. J. Appl. Physiol. 119:1463-1478.
Sports Integrity Australia. (2019). Medical information to support a TUE application: Iron infusions. Retrieved 21st March 2023 from https://www.sportintegrity.gov.au/sites/ default/files/ASDMAC%20-%20Iron%20Infusions%20Fact%20Sheet%20-%20 November%20%202019_0.pdf
Stellingwerff, T., P. Peeling, L.A. Garvican-Lewis, R. Hall, A.E. Koivisto, I.A. Heikura, and L.M. Burke (2019). Nutrition and altitude: Strategies to enhance adaptation, improve performance and maintain health: A narrative review. Sports Med. 49(Suppl 2):169- 184.
Stoffel, N.U., C. Zeder, G.M. Brittenham, D. Moretti, and M.B. Zimmermann (2020). Iron absorption from supplements is greater with alternate day than with consecutive day dosing in iron-deficient anemic women. Haematologica 105:1232-1239.
Troutt, J.S., M. Rudling, L. Persson, L. Stahle, B. Angelin, A.M. Butterfield, A.E. Schade, G. Cao, and R.J. Konrad (2012). Circulating human hepcidin-25 concentrations display a diurnal rhythm, increase with prolonged fasting, and are reduced by growth hormone administration. Clin. Chem. 58:1225-1232.
Wasserfurth, P., J. Palmowski, A. Hahn, and K. Kruger (2020). Reasons for and consequences of low energy availability in female and male athletes: Social environment, adaptations, and prevention. Sports Med. Open 6:44.