KEY POINTS
- Female athletes are at risk for low energy availability (LEA) which in the long-term impairs bone health and function with up to a 4-fold increased risk of bone stress injuries.
- Young athletes are especially vulnerable as 90% of peak bone mass development is complete by age 18, and susceptibility of the endocrine system to the effects of LEA is greatest during the early peak bone-building years.
- Carbohydrate availability may play a role independent of LEA.
- Changes to bone tissue occur slowly over time which makes early detection and reversal of changes challenging.
- Primary prevention of LEA (education and ensuring optimal nutrition to support bone health) should be emphasized.
- When impaired bone health, such as low bone mineral density or a bone stress injury is detected, best practice secondary (early screening and detection of bone health impairments) and tertiary (treatment of poor bone health preferentially via non-pharmacological interventions) prevention is important for optimal long-term health and performance support of the female athlete.
INTRODUCTION
In 2017, Bobby Clay, a then 20-year-old distance running talent from the United Kingdom, shared her personal struggles with poor bone health on several online platforms (Clay, 2017). At the age of 18, Clay received a devastating, career-ending diagnosis of osteoporosis. By the time she had noticed the first symptoms of damage, resulting from years of unintentional under-fuelling and overtraining, it was already too late. The story is devastating and unfortunately too common among female athletes. The phenomenon characterising low energy availability (LEA), menstrual dysfunction and poor bone health was identified as the Female Athlete Triad over three decades ago (Yeager et al., 1993). Recently, the concept of Relative Energy Deficiency in Sport (REDs) was introduced to acknowledge the complexity and breadth of LEA (Mountjoy et al., 2014).
Bone stress injuries (BSI) are one of the most insidious and challenging outcomes of REDs in female athletes. This is often because it is not until a BSI is diagnosed that the underlying, often prolonged and/or severe LEA, is discovered. BSI are the most common injury in athletes and two-to-four times more common among females than males with prevalence rates as high as 20% (Popp et al., 2022) depending on sport type. A single BSI can keep an athlete from sport anywhere from ~4 weeks (Hoenig et al., 2023) to 6 months (Popp et al., 2022), potentially impacting competition and performance outcomes (Raysmith & Drew, 2016). Moreover, effects extend beyond performance outcomes to psychological and social aspects of life (Gillbanks et al., 2022b).
Since BSI are usually preceded by decreases in bone mineral density (BMD) (Holtzman et al., 2022), and since any meaningful change (positive or negative) in the structural characteristics of the bone tissue can take months or years to occur, impairments to bone health can be incredibly difficult to prevent, treat and reverse. At least 90% of peak bone mass is attained by age 18 (Bailey et al., 1999), and therefore any nutritional or lifestyle challenges in the years preceding this age have the potential to risk bone health and long-term health and performance. The long-term consequences of poor bone health are obvious and severe, in the worst case risking early osteoporosis and subsequent implications later in life (Hosmer et al., 2002). Therefore, prevention and proactive steps to support optimal bone health across the female athlete’s lifespan are important for overall athlete health, well-being, and sports performance.
This Sports Science Exchange (SSE) article will explore the key nutritional challenges and opportunities to support optimal bone health in female athletes, followed by a stepwise action plan to prevent poor bone health. Here, poor bone health is defined as low BMD (age and sex-specific Z-score < -1.0 measured via Dual-energy X-ray Absorptiometry) and/ or BSI. BSI range from stress reactions to complete stress fractures (Popp et al., 2022). This SSE will colloquially use the word BSI to refer to any stress-related injury to the bone, regardless of severity. While acknowledging that BSI are multifactorial and the etiology can include several non-nutritional risk factors (training volume and type including impact vs non-impact loading, psychological stress and well-being, and lifestyle factors such as smoking), this SSE will primarily focus on nutritional aspects. The goal of this article is to provide practical guidance for female athletes and their coaches, parents, and health and performance support staff toward optimal bone health and function across the lifespan.
NUTRITIONAL CHALLENGES AND OPPORTUNITIES FOR OPTIMAL BONE HEALTH IN FEMALE ATHLETES
The bone is a highly dynamic tissue that undergoes renewal at a rate of 5 (cortical bone) to 20% (trabecular bone) per year (Fernandez- Tresguerres-Hernandez-Gil et al., 2006). This represents an ongoing opportunity but also a challenge for the female athlete to make daily choices to support optimal bone health across the lifespan. Both nutrition and physical activity play key roles in this process. More specifically, lack of nutrients to build new bone tissue as well as inadequate external load (such as high-impact physical activity) will subsequently lead to poor bone health. The following sections will discuss the main nutritional deficiencies related to the development of poor bone health (challenges) followed by practical guidelines for nutritional strategies to mitigate these outcomes (opportunities).
Energy availability
The Challenge. Problematic LEA is the single most important nutritional culprit behind impaired bone health and function in female athletes. The downstream effects of LEA are well-known and severe, culminating in a cluster of impairments known as REDs (Mountjoy et al., 2023). In terms of bone health, decreased BMD and subsequent development of BSI leading to compromised athlete availability are important considerations.
In females, several endocrine pathways (reproductive, metabolic, and thyroid hormones specifically) and markers of bone remodeling appear to be disrupted after only 4 - 5 days of Energy Availability (EA) < 30 kcal·kg fat-free mass (FFM)-1·d-1 (Ihle & Loucks, 2004; Loucks & Thuma, 2003; Papageorgiou et al., 2018). Decreases in the secretory patterns of the hypothalamic-pituitary-gonadal axis (in particular, luteinizing hormone pulsatility, and gonadotropin-releasing hormone pulsatility) result in decreased estradiol concentrations (Gordon et al., 2017). Over time, this down-regulates menstrual function (Gordon et al., 2017) and bone metabolism (Lewis et al., 2021), ultimately resulting in poor structural and functional bone health. Hypothalamic amenorrhea is a type of secondary amenorrhea, which contributes to reductions in bone mass (Cabre et al., 2022) and can be broken down into primary amenorrhea and secondary amenorrhea. Primary amenorrhea is defined by the lack of a menstrual cycle by the age of 15 in a female who has developed secondary sexual characteristics, or age 14 when no secondary sexual characteristics are present, while secondary amenorrhea is the absence of 3 or more consecutive menstrual cycles in a female with a history of normal menstruation (Cabre et al., 2022). Lack of menstruation is often the first visible sign of LEA in the female athlete and thus often used as a surrogate for LEA in observational studies.
Several studies utilizing surrogate markers of long-term LEA have consistently associated menstrual dysfunction (ranging from oligomenorrhea to amenorrhea, Williams et al., 2015) with 2 – 4 fold greater prevalence and risk of career BSI and lifetime bone fractures (Ackerman et al., 2015; Heikura et al., 2018) compared to female athletes with regular cycles. Female athletes with menstrual dysfunction often present with lower BMD, impaired bone microarchitecture and estimates of bone strength (Ackerman et al., 2011). Importantly, LEA and estrogen deficiency exert combined and independent effects on bone structural and functional health (Southmayd et al., 2017), so LEA even in the absence of menstrual dysfunction, poses a risk. This highlights the importance of looking beyond menstrual dysfunction when screening for LEA (see Secondary Prevention section). Indeed, other surrogate markers of LEA or calculations of risk scores based on clusters of REDs indicators have frequently been associated with increased risk of BSI in females. For example, studies utilizing the Female Athlete Triad Cumulative Risk Assessment tool [CRA; a scoring tool based on varying severity of low body mass index, disordered eating/eating disorders, menstrual function, BMD, and BSI history (Joy et al., 2014)] report 2 – 4 fold greater number of career BSI in athletes with moderate- to high-risk CRA scores (Tenforde et al., 2017). Furthermore, each 1-point increase in the CRA score has been reported to yield a 37% higher risk of a prospective BSI (Tenforde et al., 2022). Along with total CRA scores, restrictive eating behavior has been independently associated with a history of multiple BSI in female athletes (Gehman et al., 2022).
Studies have also investigated the effects of LEA on the prevalence (retrospective) and risk (prospective) of high- vs low-risk BSI in female athletes. High-risk BSI on the trabecular bone (femoral neck, sacrum) tend to be more severe and prolong the return to sport (Hoenig et al., 2023). In female athletes, disordered eating behavior, a BMD Z-score < -1.0, and accumulating a number of Triad risk factors have been linked to increased odds of high-risk BSI independent of menstrual status (Holtzman et al., 2022). Furthermore, females with low BMD and low body mass appear to have greater odds of future high-risk BSI (Tenforde et al., 2022).
While REDs is always preceded by LEA, it is important to highlight that not all incidents of LEA lead to REDs. Brief periods of LEA can alter hormone concentrations, and even some bone metabolic markers but these changes may not be an acute concern to bone health, or even the broader concept of REDs, in cases where LEA is brief and/ or mild in nature. Changes to the bone require the presence of severe and/or prolonged LEA, where altered hormonal profile together with direct effects on the bone over a prolonged period results in impaired bone metabolism, structure, and function. That said, athletes are encouraged to strive for adequate EA for optimal long-term health and performance outcomes as any impairment to the bone tissue will likely take a substantial amount of time to repair or restore function and at times, damage may be irreversible (Warren et al., 2002).
The Opportunity. Female athletes should ensure optimal EA to support bone health and function as part of the primary prevention (see the Primary Prevention section, and Figure 1). What is defined as optimal EA is likely to depend on each individual athlete and their personal circumstances but is likely around 40 - 45 kcal·kg FFM-1·d-1 (Loucks et al., 2011). Since calculations of EA are difficult and not exactly feasible, the best indicators for achieving and maintaining optimal EA rely on subjective and objective assessments of health, well-being, and performance. Here, regular self-assessment (by athletes) and observation (by athletes and support team) of indicators of REDs is crucial (please see Secondary Prevention section).
Carbohydrate Availability
The Challenge. Most athletes recognize the importance of carbohydrates in supporting training quality, adaptation, and performance goals (Moore et al., 2022). However, recent evidence suggests that carbohydrates may also play an energy-independent role in several health outcomes. In the context of bone health, limiting carbohydrate intake before (Scott et al., 2012), during (Sale et al., 2015) or after (Townsend et al., 2017) exercise may acutely alter the bone remodeling profile in favor of bone resorption (Sale & Elliott-Sale, 2020). A limited number of studies in female and male athletes suggest that acute (24 h) (Hammond et al., 2019) and short-term (5 d to 3.5 wk) (Fensham et al., 2022; Heikura et al., 2019) low carbohydrate diets (< 50 g/d) may also impair the bone remodeling profile. Whether these changes eventually translate into functional outcomes including decreased BMD and increased risk of BSI in athletes, remains unknown and is currently difficult to decipher based on existing evidence.
The Opportunity. Female athletes should ensure adequate daily and within-day carbohydrate availability based on the metabolic demands of training and competition. Since the exact amount will be specific to each athlete, sport, and training or competition phase, in-depth discussion is beyond the scope of this paper and the reader is referred to publications such as Moore et al. (2022) and Impey et al. (2018).
Other Nutritional Considerations
The Challenge. Dietary protein may exert a small net positive effect on female athletes’ bone mass and fracture risk, most likely via effects on anabolic pathways and calcium homeostasis (Dolan & Sale, 2019). In general, athletes appear to consume plenty of protein (Heikura et al., 2017) and therefore protein intake is unlikely to become the rate-limiting step for bone health. One common scenario where dietary protein may become compromised is during periods of LEA. While adequate protein may rescue some of the effects of LEA on the body, a higher protein intake does not seem to mitigate the impairments to bone remodeling during LEA (Murphy et al., 2021).
While detailed discussion of micronutrients is beyond the scope of this article (see Sale & Elliott-Sale, 2020), the importance of adequate calcium and vitamin D intake for optimal bone health across the lifespan and regardless of activity level deserves brief discussion. Calcium can be described as the building block required for the mineralization of new bone tissue, while vitamin D plays a role in increasing intestinal calcium absorption, promoting bone mineralization, and regulating osteocalcin activity (Sale & Elliott-Sale, 2020) For female athletes specifically, requirements tend to be somewhat higher than for male athletes. In addition, female athletes may struggle with achieving the specific requirements due to factors such as LEA, dietary restrictions (e.g., milk allergy), or following a special diet (e.g., vegetarian). A final temporary consideration for calcium comes from recent investigations on calcium intake immediately pre-exercise. Since athletes lose calcium via sweating, studies recommend ingesting calcium in the pre-exercise meal to mitigate some of the acute outcomes of calcium homeostasis (Wherry et al., 2022).
The Opportunity. Female athletes and military recruits who consumed greater than 1,500 mg/d calcium exhibited the largest reduction in BSI (Tenforde et al., 2010). It is therefore recommended that female athletes aim to consume 1,000 - 1,500 mg calcium/d and 1,000 - 2,000 IU vitamin D/d (Figure 1 and Holtzman & Ackerman, 2021). While less researched, female athletes with a high risk of or history of poor BMD/ BSI could also benefit from 1,000 mg calcium (food or supplementary sources) at least 1 h pre-exercise (Wherry et al., 2022).
PREVENTION IS THE BEST TREATMENT
Due to the nature of bone health impairments resulting from LEA, the best treatment is undeniably prevention. The following sections will discuss education and optimized nutrition (Primary Prevention) as well as early screening and detection of female athletes at risk of REDs (Secondary Prevention). The last step – Tertiary Prevention – will discuss treatment of existing issues in relation to bone health. This three-step framework is summarized in Figure 1 and based on work by Torstveit et al. (2023) in relation to prevention of REDs. Since bone health outcomes tend to be identified at later stages of REDs, the primary and secondary prevention sections will mostly discuss prevention in the context of early indicators of REDs.
Primary Prevention
Primary prevention of poor bone health consists of several proactive steps that should consider the female athlete, their support team, and the whole sports system and essentially focus on minimizing any potential for exposure to LEA that might over the long-term lead to poor bone outcomes. Here, key concepts include knowledge and its translation into behaviour. Overall, knowledge of REDs among athletes and the broader support team remains poor (Gillbanks et al., 2022a; Lodge et al., 2022). While knowledge precedes behavior change it does not necessarily guarantee it. Therefore, athletes require an understanding of and ability to take proactive steps toward reaching optimal EA and dietary strategies (Stewart et al., 2019). Here, skills (meal planning and preparation) and capacity (finances) to implement knowledge into action, are required. This can include educational interventions (Fahrenholtz et al., 2023) or one-on-one consultations with a sports dietitian. For example, a recent 7-year prospective intervention implementing nutrition education and consultation of female athletes reported reduced rates of trabecular-rich BSI (Fredericson et al., 2023). Ideally, education extends to a broader group of individuals involved in the day-to-day life of the athlete, including parents, coaches, and teachers. Due to the nature of bone health-related issues in the female athlete (challenges of early diagnosis and poor prognosis of return-to-play), high-risk groups (aesthetic or endurance sports, younger athletes undergoing peak bone growth) should receive special attention.
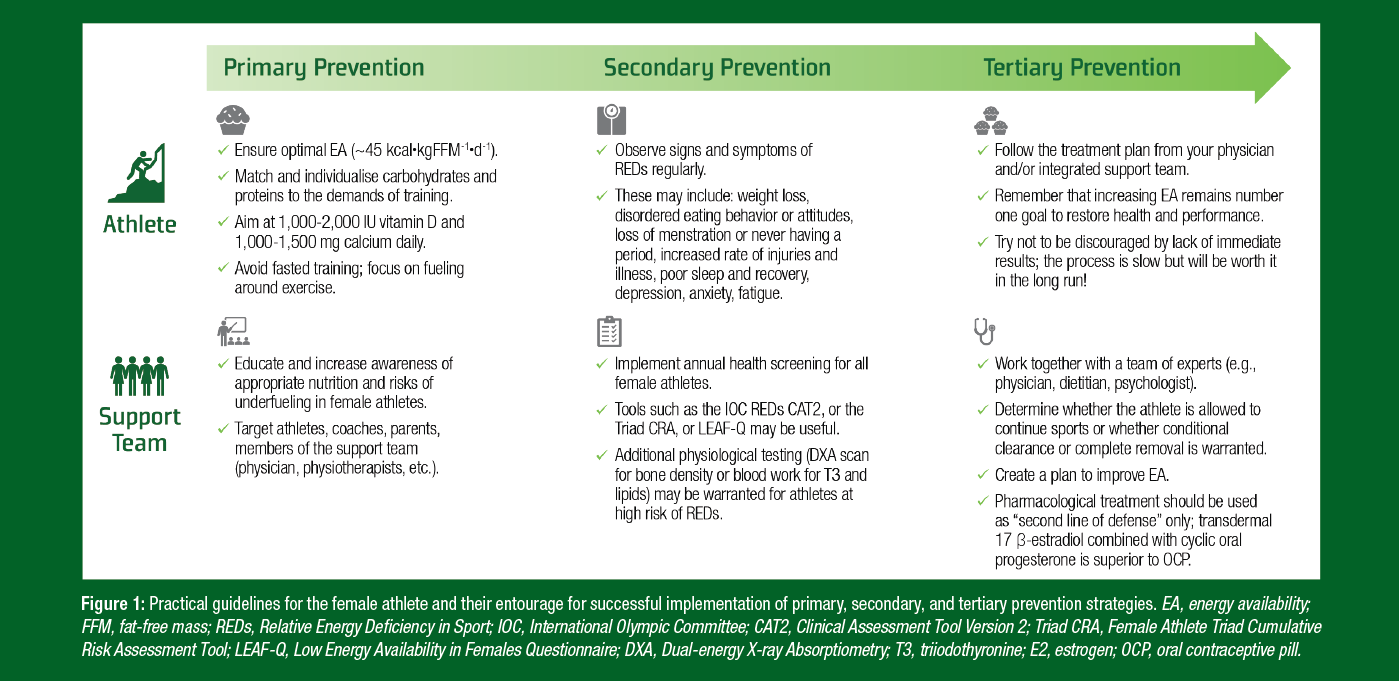
Figure 1: Practical guidelines for the female athlete and their entourage for successful implementation of primary, secondary, and tertiary prevention strategies. EA, energy availability; FFM, fat-free mass; REDs, Relative Energy Deficiency in Sport; IOC, International Olympic Committee; CAT2, Clinical Assessment Tool Version 2; Triad CRA, Female Athlete Triad Cumulative Risk Assessment Tool; LEAF-Q, Low Energy Availability in Females Questionnaire; DXA, Dual-energy X-ray Absorptiometry; T3, triiodothyronine; E2, estrogen; OCP, oral contraceptive pill.
Secondary Prevention
While primary prevention of REDs is preferable, realistically, some athletes will still sustain a BSI or demonstrate poor bone health. This highlights the importance of early identification of REDs to ensure timely intervention and prevention of further health impairments. With regards to bone, early management of REDs has the potential to completely prevent the development of poor BMD and BSI which usually take much longer to develop than some of the early consequences of REDs. Since most signs and symptoms of poor bone health (decreased BMD and BSI) are only seen once damage has already been done and significant time to recovery is likely, attention should be directed towards early signs and symptoms of REDs.
Although LEA has been mathematically defined roughly as EA < 30 kcal·kg FFM-1·d-1 (Loucks et al., 2011), in practice calculations of EA are challenged by several methodological issues (Burke et al., 2018; Heikura et al., 2022) and experts encourage assessment of clusters of physiological and psychological signs (objective measures; e.g., BMD) and symptoms (observed or self-assessed; e.g., BSI) of REDs (Heikura et al., 2022). Options for field assessment include LEA or eating disorder-specific questionnaires (Low Energy Availability in Females Questionnaire, Eating Disorder Examination Questionnaire, Brief Eating Disorder Assessment Questionnaire, etc.) as well as monitoring menstrual status, changes in body weight, increased levels of fatigue, mood instability, irritation, and depression (Stellingwerff et al., 2023). As these symptoms can be subjective and require further investigation to confirm a REDs diagnosis, more specific tools with a combination of objective (signs) and subjective (symptoms) measures may prove helpful.
The new International Olympic Committee (IOC) REDs Clinical Assessment Tool Version 2 [CAT2; (Stellingwerff et al., 2023)] is a compilation of six primary (primary or secondary amenorrhea, low BMD, history of BSI, low triiodothyronine, eating disorder/disordered eating, and deviation from growth curve) and four (oligomenorrhea, history of BSI, depression, and elevated total or low-density lipoprotein cholesterol) secondary indicators of REDs, where clusters of these indicators form the basis of traffic light risk/severity assessment (green, yellow, orange or red light category). Some of the indicators can develop after relatively brief periods of problematic LEA (e.g., decreased triiodothyronine concentrations or menstrual dysfunction); while others are only detected at a later stage (decreased BMD and/or BSI). While future studies will need to test the REDs CAT2 in practice, previous studies implementing the CRA support the idea that the presence of any indicator poses a risk to an athlete’s bone health, whereas the presence of a cluster of indicators increases the odds of BSI or poor BMD significantly (Barrack et al., 2014; Tenforde et al., 2017). Detailed discussion of screening of REDs is beyond the scope of this article and the reader is referred to the new IOC REDs CAT2 publication (Stellingwerff et al., 2023).
In line with the IOC recommendations, suggest female athletes should be screened for symptoms of REDs annually (Stellingwerff et al., 2023). Only athletes cleared to train and compete (green and yellow light) should be allowed to do so, whereas athletes with increased risk (orange and red light) should receive support in restoring health and function prior to full return to training and competition.
Tertiary Prevention
Female athletes may benefit from additional bone health interventions in the setting of recurrent BSI and/or when diagnosed with low BMD. Bone health improvements can be made through restoring regular menstrual cycles and addressing LEA in adolescent females, however, deficits in BMD may remain in some athletes (Misra et al., 2008). If normal BMD is not achieved through 6-12 months of dedicated nutritional and exercise interventions and the female athlete remains in an amenorrheic state, pharmacologic interventions can be considered (Gordon et al., 2017). When bone health interventions are considered, medical professionals should take an individualized approach to each athlete’s condition, factoring in age, severity of low BMD, BSI history, and commitment to all aspects of their care, including their nutrition and physical activity level.
Optimal estrogen levels are an important factor in achieving a healthy BMD, however different methods of delivery of exogenous estradiol have been shown to have varying effects on bone health. On one hand, the administration of estrogen-progesterone oral contraceptive pills (OCPs) has been shown to have no effect on improving BMD or reducing fracture risk (Nappi et al., 2012). Oral exogenous estradiol, like that in OCPs, has been shown to inhibit the release of insulin-like growth factor 1 (IGF-1) which may in turn reduce bone formation (Horenz et al., 2022). Unlike combined OCPs, transdermal estrogen does not affect secretion of IGF-1 (Mountjoy et al., 2018). Transdermal estrogen has been shown to improve BMD and bone microarchitecture in female athletes with functional hypothalamic amenorrhea (Ackerman et al., 2020). This is typically administered transdermally as 17β-estradiol and is taken alongside cyclic oral progesterone (Gordon et al., 2017; Mountjoy et al., 2018). One randomized control trial found increases in total hip, femoral neck, and lumbar spine BMD Z-scores in amenorrheic athletes over a 12-month period using a 100 μg transdermal 17-β-estradiol patch continuously applied twice weekly and cyclic oral micronized progesterone (200 mg) for 12 days of each month (Ackerman et al., 2020). Effects from long-term use of transdermal estrogen are not well-studied and should be used as a short-term treatment method alongside properly addressing any underlying nutritional, psychological, or exercise concerns (Gordon et al., 2017).
In rare instances, pharmacological management other than transdermal estrogen replacement can be considered when athletes present with severely low BMD and have failed nonpharmacological therapy. In these situations, athletes should be referred to an endocrinologist or an expert in metabolic bone diseases to thoroughly discuss management options and follow-up care. Bisphosphonates, an antiresorptive agent, are commonly used in osteoporosis management but are not usually recommended in athletes, due to concerns for fetal teratogenicity in women of childbearing age (Gordon et al., 2017). Further, bisphosphonates may delay BSI healing by suppressing intracortical remodeling and damage removal (Sloan et al., 2010). Recombinant parathyroid hormone 1-34 (rPTH) stimulates osteoblasts more than osteoclasts and may impact bone healing. rPTH has been shown to improve BMD by improving bone remodeling in those recovering from anorexia nervosa (Fazeli et al., 2014), however, it should only be considered in athletes with delayed fracture healing or very low BMD (Gordon et al., 2017). These medications are also not recommended for adolescents with open growth plates (Hoenig et al., 2022).
Alongside efforts to improve EA, strength training and/or plyometric activities may be another method to improve BMD in athletes. There is limited evidence in this area, however, previous studies have shown that strength training in male athletes improved BMD compared to both run training and sedentary controls (Kuikman et al., 2021). In addition, through explosive jumping or hopping motions, plyometrics may provide an advantageous, multi-dimensional loading of bone (Lambert et al., 2020).
PRACTICAL APPLICATIONS
- Nutrition education is important to ensure female athletes have the knowledge and skills to implement a diet that provides an optimal EA (roughly 40 - 45 kcal·kg FFM-1·d-1), matches daily and within-day carbohydrate availability to the demands of training, and has sufficient amounts of calcium and vitamin D to support bone health across the lifespan.
- Participation in annual REDs screening as well as regular self-assessment and observation of key indicators of REDs is important to allow for early detection and intervention or treatment of issues to prevent the long-term outcomes of reduced BMD and the development of BSI.
- Treatment should be physician-led and modified for each athlete but usually consists of improving EA as a first line of defence, potentially followed by pharmacological interventions in cases where no improvement in BMD or amenorrhea are seen despite increases in EA. Pharmacological options typically include transdermal application of 17β-estradiol together with cyclic oral progesterone, and in rare cases, other options may be warranted.
- These steps have been summarized in Figure 1.
CONCLUSIONS
Female athletes face a unique challenge whereby risks and prevalence of LEA along with other nutrition deficiencies (inadequate carbohydrate, calcium, and vitamin D intakes) are high and subsequent repercussions potentially severe due to challenges related to lack of primary prevention measures, late identification of high-risk athletes, and poor prognosis of impaired bone health outcomes. This highlights the importance of 1) proactive steps (increased education and knowledge translation into behavior) by female athletes and the whole athlete entourage to mitigate the exposure to LEA and other nutrition deficiencies; 2) early, frequent screening of all female athletes for signs and symptoms of LEA and REDs; 3) physician-led early intervention and implementation of evidence-based treatment strategies in cases of compromised bone health. Proper and coordinated implementation of these steps across the “female athlete lifespan” has the potential to lay the foundation for healthy, successful female athletes that extends positive implications well beyond their sporting careers.
The views expressed are those of the authors and do not necessarily reflect the position or policy of PepsiCo, Inc
REFERENCES
Ackerman, K.E., T. Nazem, D. Chapko, M. Russell, N. Mendes, A.P. Taylor, M.L. Bouxsein, and M. Misra (2011). Bone microarchitecture is impaired in adolescent amenorrheic athletes compared with eumenorrheic athletes and nonathletic controls. J. Clin. Endocrinol. Metab. 96:3123-3133.
Ackerman, K.E., N. Cano Sokoloff, G.D. DeNardo Maffazioli, H.M. Clarke, H. Lee, and M. Misra (2015). Fractures in relation to menstrual status and bone parameters in young athletes. Med. Sci. Sports Exerc. 47:1577-1586.
Ackerman, K.E., V. Singhal, M. Slattery, K.T. Eddy, M.L Bouxsein, H. Lee, A. Klibanski, and M. Misra (2020). Effects of estrogen replacement on bone geometry and microarchitecture in adolescent and young adult oligoamenorrheic athletes: A randomized trial. J. Bone Miner. Res. 35:248-260.
Bailey, D.A., H.A. McKay, R.L. Mirwald, P.R. Crocker, and R.A. Faulkner (1999). A six-year longitudinal study of the relationship of physical activity to bone mineral accrual in growing children: The University of Saskatchewan bone mineral accrual study. J. Bone Miner. Res. 14:1672-1679.
Barrack, M.T., J.C. Gibbs, M.J. De Souza, N.I. Williams, J.F. Nichols, M.J. Rauh, and A. Nattiv (2014). Higher incidence of bone stress injuries with increasing female athlete triadrelated risk factors: A prospective multisite study of exercising girls and women. Am. J. Sports Med. 42:949-958.
Burke, L.M., B. Lundy, I.L. Fahrenholtz, and A.K. Melin (2018). Pitfalls of conducting and interpreting estimates of energy availability in free-living athletes. Int. J. Sport Nutr. Exerc.. Metab. 28:350-363.
Cabre, H.E., S.R. Moore, A.E. Smith-Ryan, and A.C. Hackney (2022). Relative energy deficiency in sport (RED-S): Scientific, clinical, and practical implications for the female athlete. Germ J. Sports Med. 73:225-234.
Clay, B. (2017). Bobby Clay – my osteoporosis nightmare. Athl. Weekly. Retrieved May 19, 2023 from https://athleticsweekly.com/performance/bobby-clay-my-osteoporosisnightmare-70422/Dolan, E., and C. Sale (2019). Protein and bone health across the lifespan. Proc. Nutr. Soc. 78:45-55.
Fahrenholtz, I.L., A.K. Melin, I. Garthe, S.M. Hollekim-Strand, A. Ivarsson, K. Koehler, D. Logue, P. Lundstrom, S. Madigan, P. Wasserfurth, and M.K. Torstveit (2023). Effects of a 16-week digital intervention on sports nutrition knowledge and behavior in female endurance athletes with risk of relative energy deficiency in sport (REDs). Nutrients 15:1082.
Fazeli, P.K., I.S. Wang, K.K. Miller, D.B. Herzog, M. Misra, H. Lee, J.S. Finkelstein, M.L. Bouxsein, and A. Klibanski (2014). Teriparatide increases bone formation and bone mineral density in adult women with anorexia nervosa. J. Clin. Endocrinol. Metab. 99:1322-1329.
Fensham, N.C., I.A. Heikura, A.K.A. McKay, N. Tee, K.E. Ackerman, and L.M. Burke (2022). Short-term carbohydrate restriction impairs bone formation at rest and during prolonged exercise to a greater degree than low energy availability. J. Bone Miner. Res. 37:1915-1925.
Fernandez-Tresguerres-Hernandez-Gil, I., M. Alobera Gracia, M. del Canto-Pingarron, and L. Blanco-Jerez (2006). Physiological bases of bone regeneration II. The remodeling process. Med. Oral Patol. Oral Cir. Bucal. 11:51-57.
Fredericson, M., M. Roche, M.T. Barrack, A. Tenforde, K. Sainani, E. Kraus, A. Kussman, E. Miller Olson, B.Y. Kim, K. Fahy, E. Miller, E. Diamond, S. Meraz, S. Singh, and A. Nattiv (2023). Healthy Runner Project: a 7-year, multisite nutrition education intervention to reduce bone stress injury incidence in collegiate distance runners. B.M.J. Open Sport Exerc. Med. 9:e001545.
Gehman, S., K.E. Ackerman, S. Caksa, S.E. Rudolph, J.M. Hughes, M. Garrahan, A.S. Tenforde, M.L. Bouxsein, and K.L. Popp (2022). Restrictive eating and prior low-energy fractures are associated with history of multiple bone stress injuries. Int. J. Sport Nutr. Exerc. Metab. 32:325-333.
Gillbanks, L., M. Mountjoy, and S.R. Filbay (2022a). Insufficient knowledge and inapproriate physiotherapy management of Relative Energy Deficiency in Sport (RED-S) in lightweight rowers. Phys. Ther. Sport 54:8-15.
Gillbanks, L., M. Mountjoy, asnd S.R. Filbay (2022b). Lightweight rowers' perspectives of living with Relative Energy Deficiency in Sport (RED-S). PLoS One 17:e0265268.
Gordon, C.M., K.E. Ackerman, S.L. Berga, J.R. Kaplan, G. Mastorakos, M. Misra, M.H. Murad, N.F. Santoro, and M.P. Warren (2017). Functional hypothalamic amenorrhea: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 102:1413-1439.
Hammond, K.M., C. Sale, W. Fraser, J. Tang, S.O. Shepherd, J.A. Strauss, G.L. Close, M. Cocks, J. Louis, J. Pugh, C. Stewart, A.P. Sharples, and J.P. Morton (2019). Postexercise carbohydrate and energy availability induce independent effects on skeletal muscle cell signalling and bone turnover: Implications for training adaptation. J. Physiol. 597:4779-4796.
Heikura, I.A., L.M. Burke, A.A. Mero, A.L.T. Uusitalo, and T. Stellingwerff (2017). Dietary microperiodization in elite female and male runners and race walkers during a block of high intensity precompetition training. Int. J. Sport Nutr. Exerc. Metab. 27:297-304.
Heikura, I.A., A.L.T. Uusitalo, T. Stellingwerff, D. Bergland, A.A. Mero, and L.M. Burke (2018). Low energy availability is difficult to assess but outcomes have large impact on bone injury rates in elite distance athletes. Int. J. Sport Nutr. Exerc. Metab. 28:403-411.
Heikura, I.A., L.M. Burke, J.A. Hawley, M.L. Ross, L. Garvican-Lewis, A.P. Sharma, A.K.A. McKay, J.J. Leckey, M. Welvaert, L. McCall, and K.E. Ackerman (2019). A short-term ketogenic diet impairs markers of bone health in response to exercise. Front. Endocrinol. 10: 880.
Heikura, I.A., T. Stellingwerff, and J.L. Areta (2022). Low energy availability in female athletes: From the lab to the field. Eur. J. Sport Sci. 22:709-719.
Hoenig, T., K.E. Ackerman, B.R. Beck, M.L. Bouxsein, D.B. Burr, K. Hollander, K.L. Popp, T.Rolvien, A.S. Tenforde, and J.S. Warden (2022). Bone stress injuries. Nat. Rev. Dis.Primers 8:26.
Hoenig, T., J. Eissele, A. Strahl, K.L. Popp, J. Sturznickel, K.E. Ackerman, K. Hollander, S.J. Warden, K.H. Frosch, A.S. Tenforde, and T. Rolvien (2023). Return to sport following low-risk and high-risk bone stress injuries: A systematic review and meta-analysis. Br.J. Sports Med. 57:427-432.
Holtzman, B., and K.E. Ackerman (2021). Practical approaches to nutrition for female athletes. SSE #215.
Holtzman, B., K.L. Popp, A.S. Tenforde, A.L. Parziale, K. Taylor, and K.E. Ackerman (2022). Low energy availability surrogates associated with lower bone mineral density and bone stress injury site. PM & R 14:587-596.
Horenz, C., M. Vogel, K. Wirkner, U. Ceglarek, J. Thiery, R. Pfaffle, W. Kiess, and J. Kratzsch (2022). BMI and contraceptives affect new age-, sex-, and puberty-adjusted IGF-I and IGFBP-3 reference ranges across life span. J. Clin. Endocrinol. Metab. 107:e2991-e3002.
Hosmer, W.D., H.K. Genant, and W.S. Browner (2002). Fractures before menopause: A red flag for physicians. Osteoporos Int. 13:337-341.
Ihle, R., and A.B. Loucks (2004). Dose-response relationships between energy availability and bone turnover in young exercising women. J. Bone Miner. Res. 19:1231-1240.
Impey, S.G., M.A. Hearris, K.M. Hammond, J.D. Barlett, J. Louis, G.L. Close, and J.P. Morton (2018). Fuel for the work required: A theoretical framework for carbohydrate periodization and the glycogen threshold hypothesis. Sports Med. 48:1031-1048.
Joy, E., M.J. De Souza, A. Nattiv, M. Misra, N.I. Williams, R.J. Mallinson, J.C. Gibbs, M. Olmsted, M. Goolsby, G. Matheson, M. Barrack, L. Burke, B. Drinkwater, C. Lebrun, A.B. Loucks, M. Mountjoy, J. Nichols, and J.S. Borgen (2014). 2014 female athlete triad coalition consensus statement on treatment and return to play of the female athlete triad. Curr. Sports Med. Rep. 13:219-232.
Kuikman, M.A., M. Mountjoy, T. Stellingwerff, and J.F. Burr (2021). A Review of nonpharmacological strategies in the treatment of relative energy deficiency in sport. Int. J. Sport Nutr. Exerc. Metab. 31:268-275.
Lambert, C., B.R. Beck, A.T. Harding, S.L. Watson, and B.K. Weeks (2020). Regional changes in indices of bone strength of upper and lower limbs in response to high intensity impact loading or high-intensity resistance training. Bone 132:115192.
Lewis, K.J., P. Cabahug-Zuckerman, J.F. Boorman-Padgett, J. Basta-Pljakic, J. Louie, S. Stephen, D.C. Spray, M.m. Thi, Z. Seref-Ferlengez, R.J. Majeska, S. Weinbaum, and M.B. Schaffler (2021). Estrogen depletion on In vivo osteocyte calcium signaling responses to mechanical loading. Bone 152:116072. Sports Science Exchange (2023) Vol. 36, No. 241, 1- 7
Lodge, M.T., K.E. Ackerman, and J. Garay (2022). Knowledge of the female athlete triad and relative energy deficiency in sport among female cross-country athletes and support staff. J. Athl. Train. 57:385-392.
Loucks, A.B., and J.R. Thuma (2003). Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J. Clin. Endocrinol. Metab. 88:297-311.
Loucks, A.B., B. Kiens, and H.H. Wright (2011). Energy availability in athletes. J. Sports Sci. 29(Suppl 1):S7-S15.
Misra, M., R. Prabhakaran, K.K. Miller, M.A. Goldstein, D. Mickley, L. Clauss, P. Lockhart, J. Cord, D.B. Herzog, D.K. Katzman, and A. Klibanski (2008). Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1. J. Clin. Endocrinol. Metab. 93:1231-1237.
Moore, D.R., J. Sygo, and J.P. Morton (2022). Fuelling the female athlete: Carbohydrate and protein recommendations. Eur. J. Sport Sci. 22:684-696.
Mountjoy, M., J. Sundgot-Borgen, L. Burke, S. Carter, N. Constantini, C. Lebrun, N. Meyer, R. Sherman, K. Steffen, R. Budgett, and A. Ljungqvist (2014). The IOC consensus statement: beyond the Female Athlete Triad--Relative Energy Deficiency in Sport (RED-S). Br. J. Sports Med. 48:491-497.
Mountjoy, M., J.K. Sundgot-Borgen, L.M. Burke, K.E. Ackerman, C. Blauwet, N. Constantini, C. Lebrun, B. Lundy, A.K. Melin, N.L. Meyer, R.T. Sherman, A.S. Tenforde, M. Klungland Torstveit, and R. Budgett (2018). IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. Br. J. Sports Med. 52:687-697.
Mountjoy, M.L., K.E. Ackerman, D.M. Bailey, L.M. Burke, N. Constantini, A.C. Hackney, I.A. Heikura, A.K. Melin, A.M. Pensgaard, T. Stellingwerff, J. Sundgot-Borgen, M.K. Torstveit, A.U. Jacobsen, E. Verhagen, R. Budgett, L. Engebretsen, and U. Erdener (2023). The 2023 International Olympic Committee’s (IOC) consensus statement on Relative Energy Deficiency in Sports (REDs). Br. J. Sports Med. In press.
Murphy, C., L.D.D. Bilek, and K. Koehler (2021). Low energy availability with and without a high-protein diet suppresses bone formation and increases bone resorption in men: A randomized controlled pilot study. Nutrients 13:802.
Nappi, C., G. Bifulco, G.A. Tommaselli, V. Gargano, and C. Di Carlo (2012). Hormonal contraception and bone metabolism: A systematic review. Contraception 86:606-621.
Papageorgiou, M., D. Martin, H. Colgan, S. Cooper, J.P. Greeves, J.C.Y. Tang, W.D. Fraser, K.J. Elliott-Sale, and C. Sale (2018). Bone metabolic responses to low energy availability achieved by diet or exercise in active eumenorrheic women. Bone 114:181-188.
Popp, K.L., L.M. Cooke, M.L. Bouxsein, and J.M. Hughes (2022). Impact of low energy availability on skeletal health in physically active adults. Calcif. Tissue Int. 110:605-614.
Raysmith, B.P., and M.K. Drew (2016). Performance success or failure is influenced by weeks lost to injury and illness in elite Australian track and field athletes: A 5-year prospective study. J. Sci. Med. Sport 19:778-783.
Sale, C., and K.J. Elliott-Sale (2020) Nutrition and athlete bone health. SSE #201.
Sale, C., I. Varley, T.W. Jones, R.M. James, J.C. Tang, W.D. Fraser, and J.P. Greeves (2015).
Effect of carbohydrate feeding on the bone metabolic response to running. J. Appl. Physiol. 119:824-830.
Scott, J.P., C. Sale, J.P. Greeves, A. Casey, J. Dutton, and W.D. Fraser (2012). Effect of fasting versus feeding on the bone metabolic response to running. Bone 51:990-999.
Sloan, A.V., J.R. Martin, S. Li, and J. Li (2010). Parathyroid hormone and bisphosphonate have opposite effects on stress fracture repair. Bone 47:235-240.
Southmayd, E.A., R.J. Mallinson, N.I. Williams, D.J. Mallinson, and M.J. De Souza (2017). Unique effects of energy versus estrogen deficiency on multiple components of bone strength in exercising women. Osteoporos. Int. 28:1365-1376.
Stellingwerff, T., M.L. Mountjoy, W.T. McCluskey, K.E. Ackerman, A.U. Jacobsen, E. Verhagen, and I.A. Heikura (2023). The IOC relative energy deficiency in sport clinical assessment tool - version 2 (IOC REDs CAT2): A narrative review by a sub-group of the IOC consensus on Relative Energy Deficiency in Sport (REDs). Br. J. Sports Med. In press.
Stewart, T.M., T. Pollard, T. Hildebrandt, N.Y. Wesley, L.S. Kilpela, and C.B. Becker (2019). The Female Athlete Body project study: 18-month outcomes in eating disorder symptoms and risk factors. Int. J. Eat. Disord. 52:1291-1300.
Tenforde, A.S., L.C. Sayres, K.L. Sainani, and M. Fredericson (2010). Evaluating the relationship of calcium and vitamin D in the prevention of stress fracture injuries in the young athlete: A review of the literature. PM & R 2:945-949.
Tenforde, A.S., J.L. Carlson, A. Chang, K.L. Sainani, R. Shultz, J.H. Kim, P. Cutti, N.H. Golden, and M. Fredericson (2017). Association of the female athlete triad risk assessment stratification to the development of bone stress injuries in collegiate athletes. Am. J. Sports Med. 45:302-310.
Tenforde, A.S., N.B. Katz, K.L. Sainani, J.L. Carlson, N.H. Golden, and M. Fredericson (2022). Female athlete triad risk factors are more strongly associated with trabecularrich versus cortical-rich bone stress injuries in collegiate athletes. Orthop. J. Sports Med. 10:23259671221123588.
Torstveit, M.K., K. Ackerman, and N. Constantini (2023). Prevention and treatment of REDs - a narrative review by a sub-group of the IOC consensus on Relative Energy Deficiency in Sport (REDs). Br. J. Sports Med. In press.
Townsend, R., K.J. Elliott-Sale, K. Currell, J. Tang, W.D. Fraser, and C. Sale (2017). The effect of postexercise carbohydrate and protein ingestion on bone metabolism. Med. Sci. Sports Exerc. 49:1209-1218.
Warren, M.P., J. Brooks-Gunn, R.P. Fox, C.C. Holderness, E.P. Hyle, and W.G. Hamilton (2002). Osteopenia in exercise-associated amenorrhea using ballet dancers as a model: A longitudinal study. J. Clin. Endocrinol. Metab. 87:3162-3168.
Wherry, S.J., C.M. Swanson, and W.M. Kohrt (2022). Acute catabolic bone metabolism response to exercise in young and older adults: A narrative review. Exp. Gerontol. 157:111633.
Williams, N.I., H.J. Leidy, B.R. Hill, J.L. Lieberman, R.S. Legro, and M.J. De Souza (2015). Magnitude of daily energy deficit predicts frequency but not severity of menstrual disturbances associated with exercise and caloric restriction. Am. J. Physiol. 308:E29-E39.
Yeager, K.K., R. Agostini, A. Nattiv, and B. Drinkwater (1993). The female athlete triad: disordered eating, amenorrhea, osteoporosis. Med. Sci. Sports Exerc. 25:775-777.